European Pharmacopoeia 12th Edition – Online
The European Pharmacopoeia (Ph. Eur.) 12th Edition is the leading reference for official quality standards governing medicines and their ingredients throughout Europe. Published by the European Directorate for the Quality of Medicines & HealthCare (EDQM), this latest edition is available exclusively online, providing instant, user-friendly access to the standards required for pharmaceutical quality, safety, and legal compliance.
Key Features:
-
Comprehensive Content:
Covers all current monographs for active substances, excipients, and finished pharmaceutical products, plus updated general chapters and analytical methods. -
365-Day Flexible Licence:
Access the latest content for a full year from activation, ensuring your knowledge and compliance stay up to date. -
Legal Requirement:
Compliance with Ph. Eur. is essential for pharmaceutical manufacturers and related organizations to legally market products across Europe. -
Effortless Online Access:
Quickly search, navigate, and reference standards from any location—boosting productivity for regulatory, quality, and laboratory teams.
What’s New in the 12th Edition:
-
Online-Only Availability:
For the first time, the Ph. Eur. is delivered exclusively in a modern digital format—no print version—making updates and navigation faster and simpler than ever. -
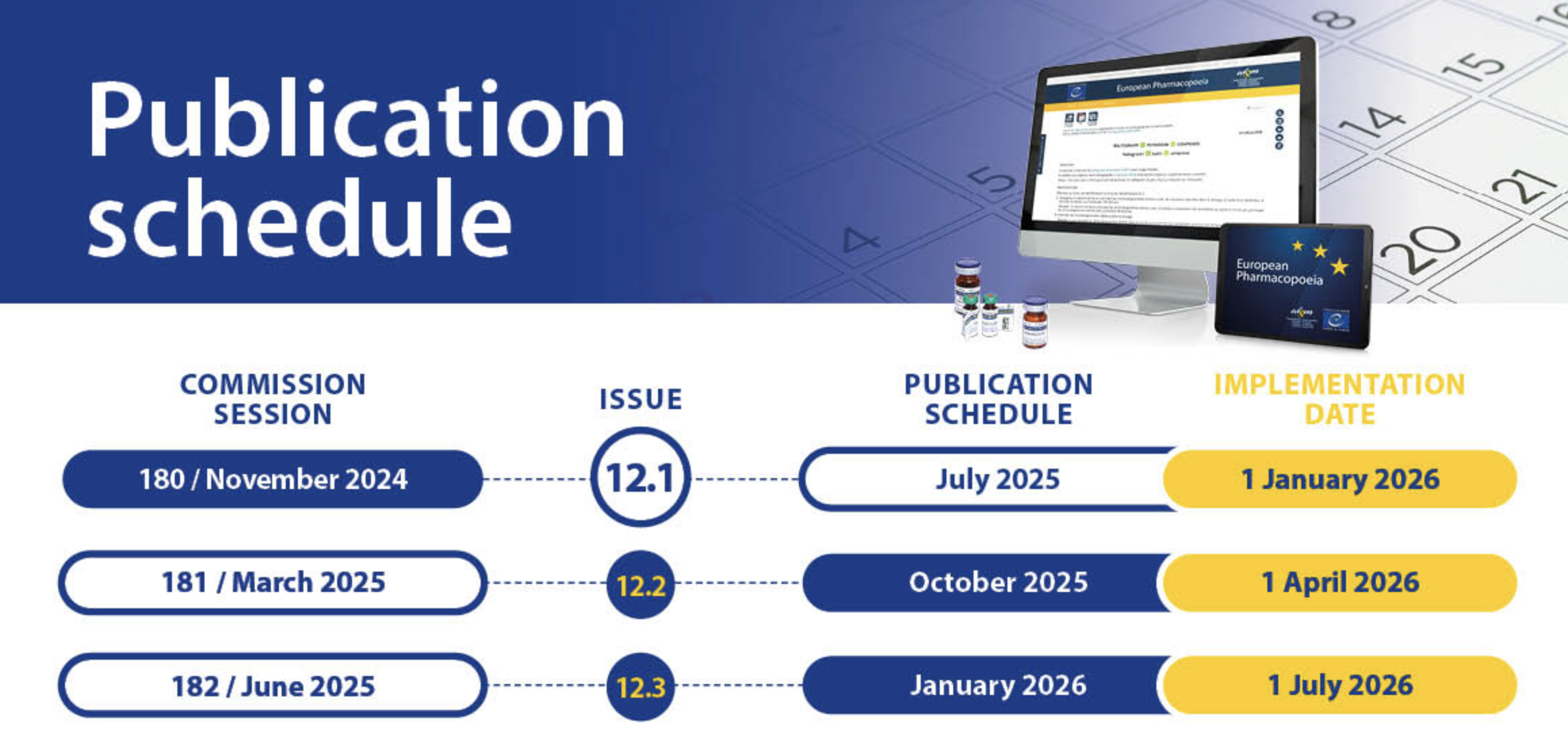
Annual Publication Cycle:
The previous three-year edition/supplement cycle has been replaced with an annual model featuring three streamlined issues per year (12.1, 12.2, 12.3), so you’re always working with the latest standards. -
Simplified Access Model:
The 12th Edition introduces a convenient, rolling 365-day licence that adapts to your organization’s needs and timing. -
Regular Standard Updates:
Each issue integrates newly adopted or revised texts and changes from the three annual sessions of the European Pharmacopoeia Commission, ensuring immediate access to the most recent regulatory expectations.
Who Should Use:
-
Pharmaceutical manufacturers and QA/QC teams
-
Regulatory affairs professionals
-
Contract laboratories and test organizations
-
Research and academic institutions
Stay ahead with the authoritative Ph. Eur. 12th Edition—your essential guide for quality control, regulatory compliance, and patient safety in the European pharmaceutical landscape.
Order your licence today for seamless, perpetual access to the industry’s gold standard.



